 Results with TECVAYLI®
Results with TECVAYLI®
TECVAYLI® was studied in 110 adults, 78% of whom had already been on at least 4 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
TECVAYLI® was studied in 110 heavily pretreated patients:
All patients have previously received a proteasome inhibitor, an immunomodulatory agent, and an anti- monoclonal antibody:
At least half of the patients had received 5 prior treatment regimens
78% of patients had 4 prior treatment regimens
experienced or better
experienced
a
experienced a
experienced
a
to treatment
experienced a
to treatment
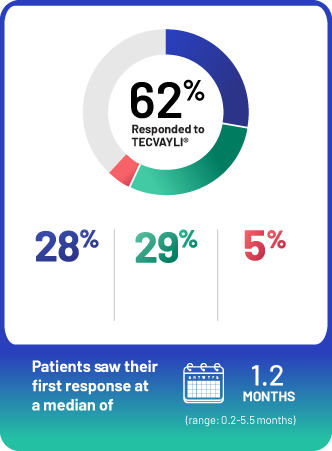
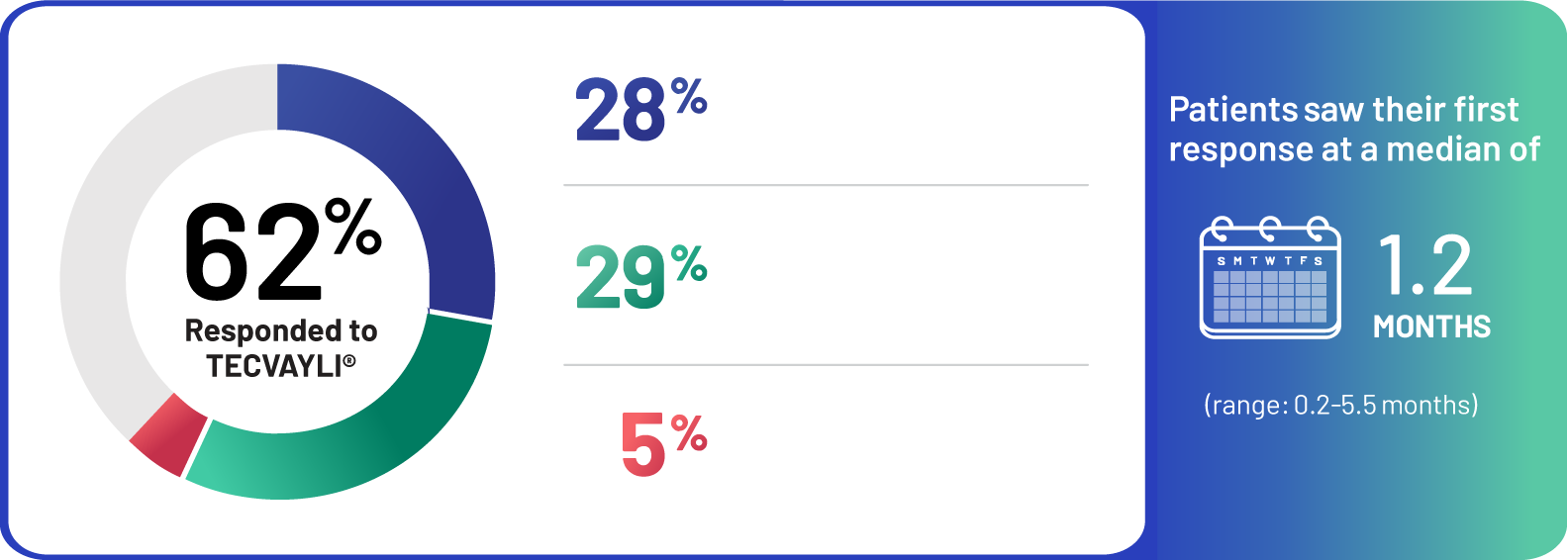
62% of people who took TECVAYLI® responded.
28% of people taking TECVAYLI® had a complete response or better to treatment. 29% of people taking TECVAYLI® had a very good partial response, and 5% had a partial response.
Median time to first response with TECVAYLI® was 1.2 months (response times ranged from 0.2 months to 5.5 months).
Talk to your doctor for more information about response.